The Extremely Complicated 1H NMR Spectrum of Ethane
It is often incorrectly assumed that simple compounds yield simple NMR spectra. The 1H NMR spectrum of ethane is such an example. The complexity arises when one takes into account the inequivalence between methyl groups in the mono 13C isotopomer which accounts for 1% of the naturally occurring ethane. In this isotopomer, one methyl group experiences a one-bond 1H - 13C coupling (1JH-C) while the other methyl group experiences a two-bond 1H - 13C coupling (2JH-C). Also, the effects of the three-bond 1H - 1H coupling (3JH-H) are exhibited in the spectrum due to the inequivalence. These couplings have a dramatic effect on the spectrum. Furthermore, there is a very small
isotope effect on the 1H chemical shifts of each methyl group due to the presence of 13C vs 12C. This effect however, is very small (~0.002 ppm) and has very little effect on the spectrum. The left panel of the figure below shows a simulation of the 1H NMR spectrum of the 12CH3-12CH3 which accounts for 99% of naturally occurring ethane. As expected, the spectrum is a singlet as both methyl groups are equivalent to one another. The middle panel of the figure shows a simulation of the 1H NMR spectrum of the 13CH3-12CH3 isotopomer which accounts for 1% of naturally occurring ethane. In this case the spectrum is extremely complex due to the 1JH-C , 2JH-C and 3JH-H coupling. The panel on the right shows a simulation of a scaled up representation of what one would expect for naturally occurring ethane.
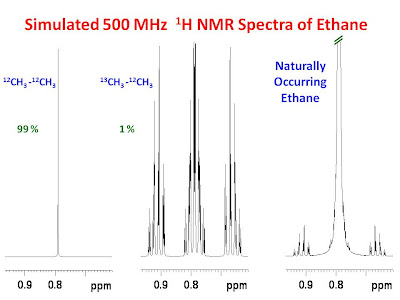
The parameters for the simulations are as follows: ??H between -12CH3 and -13CH3= 0.002 ppm, 1JH-C = 125 Hz, 2JH-C = -4.67 Hz, 3JH-H = 8 Hz and LB = 0.5 Hz.



Source:
University of Ottawa NMR Facility Blog